The information here in is intended solely as a guide for registered health professionals:
SAABI’s Information Guide for Health Professionals: Prescribing Nicotine Delivery Systems (ENDS)
The Licensee is Licensed to: Supply By Wholesale Poisons and / or Redistricted Substances for Therapeutic Use

SAABI AU PTY LTD:
Saabi holds the distinguished honour of being the sole product listed on the Australian Register of Therapeutic Goods (ARTG) with a versatile range designed specifically for step-down therapy, facilitating effective titration to aid patients in reducing nicotine consumption.
Introduction:
We at SAABI appreciate your dedication to the well-being of your patients. Our Nicotine Delivery Systems (ENDS) have been meticulously designed to support adult smokers in transitioning away from combustible tobacco products. This guide aims to provide you with essential information on SAABI’s products, highlighting their role in a step-down program for nicotine dependence and assisting you in making informed decisions when prescribing for your patients.
“Electronic cigarettes have generated a lot of misunderstanding in both the public health community and the popular press since their introduction over a decade ago. These misunderstandings discourage some people from using e-cigarettes as a stop smoking tool. Fortunately, more and more evidence is emerging and provides further clarity. With support from Cancer Research UK, we search for new evidence every month as part of a living systematic review. We identify and combine the strongest evidence from the most reliable scientific studies currently available.”
Dr Jamie Hartmann-Boyce, Associate Professor at the University of Oxford, Editor of the Cochrane Tobacco Addiction Group
COCHRANE
Why SAABI:
- SAABI’s Naturally Extracted Tobacco products are crafted for individuals seeking an alternative to traditional tobacco consumption.
- Developed for mature individuals with sophisticated tastes, our step-down program facilitates a controlled transition away from combustible tobacco.
- The program includes pods (cartridges) with varying nicotine strengths: 2%, 1.3%, and 0% (No Nicotine).
- Prescriptions enable you to prescribe a defined dose and duration, promoting a tailored approach to nicotine reduction.
Clinical Considerations:
- The RACGP’s guidelines on smoking cessation acknowledge that nicotine-containing e-cigarettes may be a reasonable intervention for individuals who have not succeeded with approved pharmacotherapies but express a desire to quit smoking.
- Caution is advised in deciding whether prescribing nicotine for inhalation aligns with the patient’s best interests, emphasizing the importance of informed decision-making.
Clinical Evidence:
- A randomized trial comparing e-cigarettes and nicotine replacement therapy (NRT) demonstrated that e-cigarettes were more effective at producing smoking cessation.
- The trial included 886 smokers attending stop-smoking services in England, with the e-cigarette group achieving an 18.0% biochemically proven cigarette abstinence rate at one year, compared to 9.9% in the NRT group.
- It’s noteworthy that among those who remained smoke-free at one year, 80% in the e-cigarette group continued using e-cigarettes, indicating their potential for sustained smoking cessation.
COCHRANE
Legislative Landscape and SAABI’s Compliance:
- SAABI operates in compliance with the evolving regulations on vapes in Australia.
- As of 1 January 2024, disposable vapes can no longer be imported, while reusable vapes must adhere to specific requirements for continued lawful supply.
- SAABI’s products align with the Therapeutic Goods (Standard for Therapeutic Vaping Goods) Order 2021 (TGO 110), focusing on smoking cessation and nicotine dependence management.
TGA VAPE HUB
Prescription Pathways:
- Health professionals can prescribe SAABI’s products through authorized channels, including the Authorised Prescriber (AP) Scheme, Special Access Scheme (SAS), and SAS C pathway.
- The AP Scheme allows medical practitioners to become authorized prescribers without the need for ethics committee approval, streamlining the prescription process.
AP PATHWAY
SAS and SAS C PATHWAY
Advice for Health Professionals:
- Consider patient-specific factors, including nicotine concentration and product type, when prescribing SAABI’s products.
- Stay informed about the evolving legislative landscape, ensuring compliance with updated standards and requirements.
- Encourage patients to report any adverse effects promptly and educate them about the potential risks, including accidental ingestion or exposure.
RACGP ADVICE
We strongly encourage consumers and health practitioners to report any suspected side effects related to vapes.
The TGA has an important role in monitoring the safety of ‘unapproved’ goods. Reporting side effects and problems helps us to understand the safety of a product. We investigate significant safety concerns as part of ensuring product safety in the Australian community.
Conclusion: We believe that SAABI’s Nicotine Delivery Systems play a valuable role in harm reduction and smoking cessation. This guide serves as a comprehensive resource to support health professionals in prescribing ENDS responsibly. SAABI remains committed to providing high-quality products that align with regulatory standards and contribute to a healthier, tobacco-free future.
For further information, please contact SAABI or refer to the Medicinal cannabis hub for additional resources on cannabis vapes, which are subject to separate regulations.
MEDICINAL CANNABIS HUB
WARNING: This product may be hazardous to health and is intended for use by adult smokers. Keep out of reach of children and pets. SAABI products are not suitable for use by: persons under the age of 18+ or 21+ Depending on your country/region, pregnant or breastfeeding women, or persons who are sensitive or allergic to nicotine, and should be used with caution by persons with or at a risk of an unstable heart condition or high blood pressure.
All SAABI® Products are manafactured under or meet the below criterion requirements so as to satisfy the relevant body.

SAABI NZ PTY LIMITED AND ITS SUBSIDIARIES, ATTEST NO CLAIM TO ANY THERAPEUTIC BENEFIT, IN RELATION TO ITS PRODUCTS OR SERVICES
Prescribing E-Cigarettes with Vaporiser Nicotine: A Step-by-Step Guide:
If, in consultation with your patient, you determine that e-cigarettes may be a beneficial tool to aid smoking cessation, you can follow the steps below:
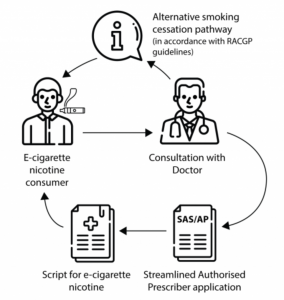
- Availability through Anspec or Local Pharmacies Nationwide:
- Arrange the acquisition of vaporiser nicotine through Anspec or your local pharmacy, which offers countrywide availability and facilitates the importation of products for smoking cessation purposes.
- The pharmacy or medical supply business will import vaporiser nicotine specifically for the named patient upon submission of a valid prescription.
- Application to the Therapeutic Goods Administration (TGA):
- Alternatively, health practitioners can apply online to the Therapeutic Goods Administration (TGA) for approval to supply goods under the Special Access Scheme B or seek authorization as an authorized prescriber.
- The TGA provides a dedicated web page with step-by-step instructions for making an application to prescribe e-cigarettes containing vaporiser nicotine.
- Special Access Scheme B (Category B) Application Process:
- Category B is an application pathway accessible when the patient does not fit the Category A definition, and the therapeutic good is not authorized for supply under the SAS Category C notification pathway.
- Health practitioners must submit a completed application with three patient identifiers, patient diagnosis and indication, product details, and prescriber details.
- The application should include a clinical justification for the use of the product, outlining why a therapeutic good currently included in the Australian Register of Therapeutic Goods (ARTG) cannot be used for the specific patient in their circumstances.
- Online Submission System for Simplified Processes:
- The TGA has introduced an online system to facilitate the electronic submission of Special Access Scheme (SAS) applications and notifications.
- This system has been updated to allow the submission of Authorised Prescriber (AP) applications, reducing administrative burden on health practitioners.
- Utilizing the online system not only streamlines the application process but also provides users with additional benefits to manage SAS applications and notifications efficiently.
Important Considerations:
- Health practitioners should carefully assess the patient’s eligibility and clinical need for e-cigarettes containing vaporiser nicotine.
- Prescriptions should adhere to regulatory requirements, and the importation or supply should be strictly for therapeutic purposes under appropriate channels.
- Stay informed about the evolving legislative landscape and TGA guidelines to ensure compliance with updated standards and requirements.
This guide aims to empower health practitioners with clear and concise steps when considering the prescription of e-cigarettes with vaporiser nicotine for smoking cessation. For further information or assistance, refer to the TGA’s dedicated web page or contact SAABI.
All prescriptions need to include:
Prescriptions are non-PBS and can be written by hand. If using medical software, you can create a template for computerised scripts using best practice methodologies.
BEST PRACTICE
It is recommended that Patients Prescription’s be kept in a safe place in case requested by authorities. It is a good idea to keep a photo of the prescription on a smartphone.
EXAMPLE ONLY
1. Nicotine concentration:
- (2% or 1.3% , 0% No Nic) LIQUID VAPORISOR NICOTINE
2. Number of Cartridge’s for defined period:
- 120 Cartridge’s in 92 days
3. Number of repeats:

CMI (Consumer Medicine Information):
CONSUMER MEDICINE INFORMATION(unregistered)
Printable Patient Information Leaflet:
PRINTABLE PATIENT INFORMATION LEAFLET
Material Safety Data Sheets:
MDSD DEVICE
MSDS CLASSIC AMERICAN
MSDS FRESH MINT
MSDS LIGHT MAPLE
MSDS TRUE APPLE
END
For The Australian Pharmaceutical Industry:
Guidance for Pharmacists: Dispensing Vapes for Smoking Cessation
The Licensee is Licensed to: Supply By Wholesale Poisons and / or Redistricted Substances for Therapeutic Use

SAABI AU PTY LTD:
This comprehensive guide is intended to equip pharmacists with essential information regarding the sourcing and dispensing of vapes for smoking cessation or the management of nicotine dependence. It covers crucial aspects such as regulatory changes, importation controls, and pathways for dispensing unapproved vapes.
SAABI expresses sincere gratitude for your thoughtful consideration of our Nicotine Delivery Systems (ENDS).
SAABI Distribution Limited are the sole distributors of SAABI Vaporised Nicotine Delivery System®
This strategic decision aligns with our dedication to positioning SAABI products in accordance with the new Australian legislation. It reflects our unwavering commitment to upholding exceptional quality and ethical standards. Australian customers now have the opportunity to consult with their physicians regarding their requirements, and doctors can prescribe SAABI nicotine delivery systems, readily available for dispensing at local pharmacies.
Overview:
- Prescription Requirement: Vapes containing nicotine can only be supplied with a valid prescription, ensuring they are dispensed exclusively in pharmacy settings.
- Reforms to Vape Regulation: New regulations aim to enhance controls on vape importation, manufacture, and supply, prioritizing public safety, especially among young people.
Vape Importation Controls:
- Effective Dates: Stricter import controls for vapes were initiated on January 1, 2024, with further measures starting on March 1, 2024.
- Disposable Vapes: As of January 1, 2024, the importation of all disposable vapes, regardless of nicotine content, is banned. Exceptions exist for disposables meeting TGA requirements, allowing lawful supply in pharmacies with a prescription.
Unapproved Vapes Dispensing:
- Pathways: Unapproved vapes may be dispensed through the Authorised Prescriber (AP) scheme, Special Access Scheme Category B (SAS B), and starting January 1, 2024, the Special Access Scheme Category C (SAS C).
- Prescription Verification: Pharmacists may dispense vapes with evidence of AP approval, SAS B approval, or adherence to the new SAS C pathway, ensuring compliance with state or territory restrictions.
Advertising and Promotion:
- Prohibitions: Advertising therapeutic goods, including vapes, is generally prohibited in Australia. Legislative changes in 2024 will further restrict vape advertising with limited exceptions.
Vaping Device Requirements:
- Framework Inclusion: From March 1, 2024, all vaping devices for use with therapeutic vaping substances must comply with specific standards outlined in TGO 110.
- Compliance Certification: Devices should adhere to ISO certifications, overseas regulator approvals, or Essential Principles for medical devices.
State and Territory Regulations:
- Additional Requirements: Pharmacists must comply with state and territory legislation, including licensing and notification requirements, when dispensing therapeutic vapes.
Reporting and Safety:
- Reporting Side Effects: Encouragement for patients and health practitioners to report suspected side effects related to vapes, aiding the TGA in monitoring product safety.
- Breaches and Questionable Practices: Reporting mechanisms are in place for individuals to notify perceived breaches or questionable practices related to the importation, manufacture, supply, export, or advertising of vapes.
We strongly encourage consumers and health practitioners to report any suspected side effects related to vapes.
The TGA has an important role in monitoring the safety of ‘unapproved’ goods. Reporting side effects and problems helps us to understand the safety of a product. We investigate significant safety concerns as part of ensuring product safety in the Australian community.
Child Poisoning Risks:
-
Accidental ingestion of, or exposure (such as through the skin or eyes) to, vapes can have toxic, and sometimes severe, effects. When dispensing vapes, you should advise your patient of the risk of accidental child poisoning if the container is left open in the process of refilling/mixing of vapes (where relevant) and/or if vapes are used in vaping devices without child-resistant safety features (including where a child is able to suck on the vaping device).
Consumers should seek urgent medical attention if they think that they, or anyone else, may have been exposed to or ingested a vaping product. Emergency services can be contacted by calling 000 and the Poisons Information Centre can be contacted by calling 131 126.
Dispensing E-Cigarettes with Vaporiser Nicotine: A Step-by-Step Guide:
This guide is designed to provide pharmacists with a step-by-step approach to sourcing and dispensing vapes for smoking cessation or nicotine dependence management. Follow these steps to ensure compliance with regulations and the safety of patients.
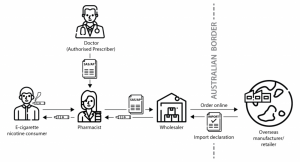
Step 1: Prescription Requirement
- Ensure that consumers must have a valid prescription for all purchases of vapes containing nicotine.
- Emphasize that the dispensing of vapes with nicotine is restricted to pharmacy settings, and it is illegal for other retailers to sell them, even with a prescription.
Step 2: Stay Informed on Regulatory Changes
- Keep abreast of regulatory changes regarding vape importation, manufacture, and supply.
- Be aware of the effective dates of new regulations, with stricter import controls initiated on January 1, 2024, and additional measures starting on March 1, 2024. Awareness: Pharmacists should educate patients about the risk of accidental ingestion or exposure to vapes, emphasising the importance of child-resistant safety features.
Step 3: Navigate Disposable Vape Regulations (From January 1, 2024)
- Understand the ban on the importation of all disposable vapes, irrespective of nicotine content.
- Recognize exceptions for disposable vapes meeting TGA requirements, allowing lawful supply in pharmacies to patients with a prescription.
Step 4: Dispensing Unapproved Vapes
- Familiarize yourself with the pathways for dispensing unapproved vapes, including the Authorised Prescriber (AP) scheme, Special Access Scheme Category B (SAS B), and starting January 1, 2024, the Special Access Scheme Category C (SAS C).
- Verify prescriptions for vapes with evidence of AP approval, SAS B approval, or adherence to the new SAS C pathway.
Step 5: Extemporaneous Compounding
- Comply with relevant compounding standards when extemporaneously compounding therapeutic vaping substances.
- Understand the responsibilities as the Australian sponsor for compounded products, ensuring conformity to TGO 110 requirements and maintaining records demonstrating compliance.
Step 6: Advertising and Promotion Compliance
- Acknowledge the prohibition of advertising therapeutic goods, including vapes, with legislative changes in 2024 further restricting vape advertising.
- Ensure that information shared between healthcare practitioners and patients during consultation is not subject to advertising rules.
Step 7: Vaping Device Requirements (From March 1, 2024)
- Prepare for changes in the therapeutic goods framework, making all vaping devices subject to compliance with specific standards outlined in TGO 110.
- Verify that devices adhere to ISO certifications, overseas regulator approvals, or Essential Principles for medical devices.
Step 8: State and Territory Regulations
- Understand and comply with state and territory legislation, including licensing and notification requirements, when dispensing therapeutic vapes.Educate patients about the risks of accidental ingestion or exposure to vapes, especially concerning child-resistant safety features.
Step 9: Reporting and Safety Measures
- Encourage patients and health practitioners to report suspected side effects related to vapes, contributing to product safety monitoring.
- Understand reporting mechanisms for notifying perceived breaches or questionable practices related to vapes.
Step 10: Child Poisoning Risks Awareness
- Educate patients about the risks of accidental ingestion or exposure to vapes, especially concerning child-resistant safety features.
By following these steps, pharmacists can navigate the complex landscape of vape regulations, ensuring patient safety and compliance with evolving standards. Stay informed about updates from relevant authorities to adapt practices accordingly
TGA VAPE HUB PHARMACY
CMI (Consumer Medicine Information):
CONSUMER MEDICINE INFORMATION(unregistered)
Printable Patient Information Leaflet:
PRINTABLE PATIENT INFORMATION LEAFLET
Material Safety Data Sheets:
MDSD DEVICE
MSDS CLASSIC AMERICAN
MSDS FRESH MINT
MSDS LIGHT MAPLE
MSDS TRUE APPLE
END
If for any reason you require further information or require assistance you can Contact our Customer Service Team via the Contact Page:
SAABI CONECT
WWW.MYSAABI.COM
All SAABI® Products are manafactured under or meet the below criterion requirements so as to satisfy the relevant body.

SAABI NZ PTY LIMITED AND ITS SUBSIDIARIES, ATTEST NO CLAIM TO ANY THERAPEUTIC BENEFIT, IN RELATION TO ITS PRODUCTS OR SERVICES
SAABI NZ PTY Limited 7855847 NZBN: 9429047848887 PO Box 210107, Laurence Stevens Drive, Auckland, 2154, New Zealand.






